Abstract
Introduction: Sickle cell disease (SCD) patients have a high rate of sudden death at early age. Median age of death has been reported to be 42 years in males and 48 years in females in a 1994 study involving over 3500 patients. Previously, Manci et al reviewed autopsies of 306 deceased SCD patients over 1929-1996. Sepsis was the most common cause of death and 40.8% patients died suddenly and unexpectedly, many within 24 hours of presentation for acute pain crisis. Cardiopulmonary causes have been attributed to the high early mortality with pulmonary hypertension as a known factor to confer a high risk of death. In a study at our institution in 2010, Kamdar et al reported that about half of the patients died suddenly, mostly from cardiopulmonary causes. We conducted a follow up study to evaluate change in trends of causes of mortality over the next 10 years as compared to our previously reported cohort.
Methods: We conducted a single institution retrospective chart review from ECU Sickle Cell Comprehensive Clinic comparing the median age of death, causes of death and other factors for patients who died between December 1998 and 2010 (cohort A, previously reported by Kamdar et al) and those who died between 2010 and 2019 (cohort B). Additionally, we evaluated changes in trends for cardiopulmonary deaths and possible contributing factors. Cause of death was determined by chart review and based on death certificates.
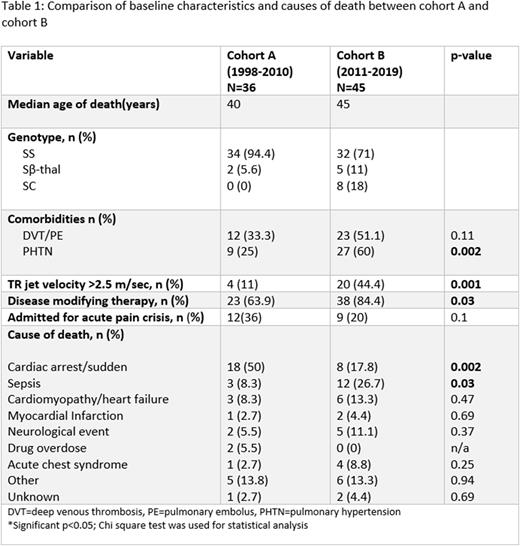
Results: Of about 350 patients following at our clinic, we identified 81 patients with sickle cell disease who died between 1998 to 2019. Amongst these, 36 patients died between 1998-2010 (cohort A) and 45 patients died between 2010-2019 (cohort B). Genotypes were Hb SS 94.4%, S b-thal 5.6% in cohort A and Hb SS 71%, Sb-thal 11% and Hb SC in 18% in cohort B. Median age of death increased from 40 years in cohort A to 45 years in cohort B. Patients from cohort A more frequently died a sudden death or cardiopulmonary arrest (50% vs 17.8%, p=0.002) and less frequently from sepsis (8% vs 26.7%, p=0.03) as compared to those in Cohort B. Disease modifying therapy use had increased from 63.9% to 84.4% (P=0.03) in these patients. In cohort B, more patients had diagnosed pulmonary hypertension (60% vs 25%, p=0.002) or had TR jet velocity >2.5 m/s (44.4 vs 11%, p=0.03). From cohort B, 40% (18 of 45) patients were following at the ECU pulmonary hypertension clinic which was started in 2009 and 33% (n=15) were on treatment for pulmonary hypertension. Other causes of death (cohort A vs B) with statistically nonsignificant differences included acute chest syndrome (2.7% vs 8.8%), heart failure/cardiomyopathy (8.3 vs 13.3%), myocardial infarction (2.7 vs 4.4%), neurological event (5.5 vs 11%), and drug overdose (5.4 vs 0%) (Table 1).
Conclusion: Comparing our two cohorts, we noted an increase in the median age of death from 40 years to 45 years. We postulate that this increase in the survival is in part related to the increased availability and prescribing of disease modifying therapies for SCD as well as increased recognition and treatment of pulmonary hypertension after inception of the pulmonary hypertension clinic at our institution. The rate of sudden cardiopulmonary arrests and sudden deaths decreased significantly which likely is the most important factor in the increase in median age of death, however, the reduction in drug overdoses as a cause of death may also have contributed. Our study is limited by the fact that it is retrospective and from a single institution, but we feel our data is compelling enough to warrant a larger prospective multi-center analysis that would focus on the impact of new disease modifying therapies.
Disclosures
Liles:Vifor: Other: presently and the Sub I or PI on trials; Biogene: Other: presently and the Sub I or PI on trials; Novartis: Other: presently and the Sub I or PI on trials; Takeda: Other: presently and the Sub I or PI on trials; Shire,: Other: presently and the Sub I or PI on trials; Incye,: Other: presently and the Sub I or PI on trials; Apellis: Other: presently and the Sub I or PI on trials; Sun Pharma: Other: presently and the Sub I or PI on trials; Momenta pharmaceuticals: Other: presently and the Sub I or PI on trials; PiCori: Other: I also am the Site Sub I for 2 PiCori grants in sickle cell anemia..
Author notes
∗Asterisk with author names denotes non-ASH members.